Abstract
Introduction: This prospective randomised trial tested whether FDG PET-CT after 2 months of chemotherapy could be used to guide subsequent treatment for patients with classical Hodgkin lymphoma (HL) requiring a full course of chemotherapy. We have previously reported the safety of de-escalation of therapy, and now present the results of longer follow up, in particular the late consequences of therapy modulation.
Methods: Adult patients (pts) with newly diagnosed HL (Ann Arbor stages IIB-IV, or IIA with bulk or ≥ 3 involved sites) underwent paired baseline and interim PET-CT scans after 2 cycles of ABVD chemotherapy (PET2). Images were reviewed centrally using the 5-point scale as negative (1-3) or positive (4-5). Pts with negative scans were randomised to ABVD or AVD, omitting bleomycin, for 4 further cycles. The primary endpoint was non-inferiority of 3-year progression free survival (PFS). Pts with positive scans proceeded to intensification with either BEACOPP-14 or escalated BEACOPP (centre choice). Radiotherapy (RT) was permitted, but not advised for pts with interim negative scans, irrespective of baseline bulk or residual masses.
Results: 1201 eligible pts received treatment. The median follow up is 87.2 months (IQR 63.0 - 104.0). The overall PFS at 7 years is 78.2% (95% CI 75.6 - 80.5) and overall survival (OS) 91.6% (95% CI 89.7 - 93.2). For pts below age 60 years 7yr PFS was 80.5% (95% CI 77.9 - 82.8) and OS 94.0% (95% CI 92.3 - 95.4). For pts with stage III/IV disease 7yr PFS was 73.4% (95% CI 69.7 - 76.8) and OS 88.7% (95% CI 85.7 - 91.0). In total 96 pts have died: 35 of HL; 22 of second malignancies; 20 of causes related to treatment. There have been a total of 23 late events beyond 5 years: 8 recurrences and 15 deaths in remission. Fifty-eight second malignancies were reported (excluding non-melanoma skin cancers and cervical intraepithelial neoplasia); 38 occurred without progression of HL, but only 3 in pts who received BEACOPP. Equal numbers occurred in pts treated with ABVD or AVD. The cumulative incidence of second malignancies at 7 years was 3.7% (95% CI 2.6 - 5.4) for pts receiving ABVD/AVD and 1.3% (95% CI 0.3 - 5.1) for those escalated to BEACOPP.
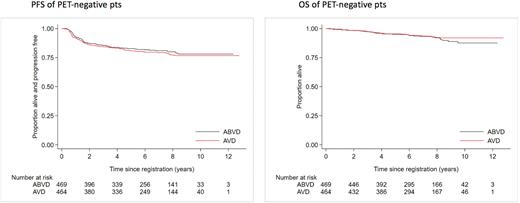
Following a negative PET2, 933 pts were randomised to continue ABVD or AVD. The -1.3% difference in 3yr PFS (95% CI -4.7 to 3.8) remains within the predefined non-inferiority margin of 5%. PFS at 7 years for ABVD was 81% (95% CI 76.9 - 84.4), and for AVD 79.2% (95% CI 75.1 - 82.8), HR: 1.10 (95%CI 0.82 - 1.47) (Figure). Subgroup analyses by stage, International Prognostic Score, presence of bulky disease and B-symptoms did not indicate significant differences in PFS by treatment arm. OS in the PET-negative group was 93.3% at 7 years (95% CI 91.3 - 94.9) with no significant difference between the arms (Figure). Among 172 pts with a positive PET2, 7 year PFS was 65.9% (95% CI 58.1 - 72.6) and 7 year OS 83.2% (95% CI 76.2 - 88.3).
Conclusion: With extended follow-up, these results reliably exclude a 5% inferior 3 year PFS following de-escalation after a negative interim PET-CT, with no evidence of a later divergence. For those with a positive PET2, escalated therapy with BEACOPP is effective and safe, with no evidence of an increase in second malignancies by comparison with the group who received ABVD/AVD.
Disclosures
Luminari:Roche: Consultancy; Janssen: Consultancy; Gilead/Kite: Consultancy; BMS/Celgene: Consultancy; Regeneron: Consultancy; Genmab: Consultancy. Trotman:Beigene: Research Funding; Roche: Research Funding; Cellectar: Research Funding; Takeda: Research Funding; BMS: Research Funding; PCYC: Research Funding; Janssen: Other: clinical trials. Molin:Roche: Honoraria; Takeda: Honoraria; MSD: Honoraria; BMS: Honoraria. Barrington:Bristol Myers Squibb international: Research Funding; Astra Zeneca: Research Funding; Amgen Ltd: Research Funding; Novartis: Research Funding; Pfizer: Research Funding; Takeda: Research Funding. Radford:The University of Manchester and Christie Hospital NHS Foundation Trust: Current Employment; ADC Therapeutics: Consultancy, Current equity holder in private company, Current holder of stock options in a privately-held company, Honoraria, Speakers Bureau; Takeda: Consultancy, Research Funding, Speakers Bureau; BMS: Consultancy, Honoraria; Kite Pharma: Consultancy; Astrazenca: Current equity holder in private company, Current holder of stock options in a privately-held company. Kirkwood:Kite: Consultancy, Honoraria. Johnson:Novartis: Honoraria; MorphoSys: Honoraria; Kymera: Honoraria; Kite Pharma: Honoraria; Incyte: Honoraria; Genmab: Honoraria; Celgene: Honoraria; BMS: Honoraria; Boehringer Ingelheim: Consultancy; Oncimmune: Consultancy; Janssen: Consultancy; Epizyme: Consultancy, Research Funding; Takeda: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal